Picture and bio information from: http://www.sickkids.ca/AboutSickKids/Directory/People/G/Brenda-Gallie.html

INTRO: Dr. Brenda Gallie is an ophthalmologist at The Hospital for Sick Children, who specializes exclusively in retinoblastoma. Her research has led her to the discovery of fundamental principles of tumor suppressor genes and the regulation of activity of the retinoblastoma (RB) gene and protein. Having developed a cost effective and highly sensitive test for identifying RB gene mutations and continuing to make great contributions with her clinical and basic research, Dr. Gallie serves as a world leader in retinoblastoma research and treatment. In 2018, she received the Helen Keller Prize in Vision Research, one of the most prestigious awards in the field of ophthalmology.
In the following interview, Dr. Gallie shares the story of her patient, “Zoe Brave,” the first child to be treated with the Chemoplaque, an episcleral implant for Topotecan delivery that has been in the process of being developed for over twenty years. She walks Know The Glow® through the patient’s case using her online database, DEPICT HEALTH, a database she started herself which contains online records of patients’ treatment histories and current status.
Q: What is your specialty? What do you like most about what you do and what is the most challenging?
Dr. Brenda Gallie: My specialty is Ophthalmic Oncology. I only do Retinoblastoma now, but before 1985, I did all eye cancers at first and then I became focused on Retinoblastoma exclusively. Officially, I see patients 30% of my time. In reality that ends up being variably anywhere from 30-60% at the Hospital for Sick Kids. The rest of my time is spent on global Retinoblastoma. So all my work is focused on Retinoblastoma and research to gain an evidence base as to the care we give the children which is quite missing in Retinoblastoma. We need an official, well-documented evidence of what treatment to use under which circumstances. I like that Retinoblastoma is a pilot for all sorts of issues in cancer and healthcare because it is rare and special enough that we keep discovering things that then can be applied broadly. The most challenging thing is bureaucracy which I am not very patient with.
Q: Recently, one of your patients was the first to be treated with Episcleral Topotecan. What can you share about this case and can you please tell us a little about your Depict RB database?
On DePict Database: The original purpose of the database in the year 2000 was because I have a poor memory. I couldn’t keep track in my memory the exact history of the child I was next bringing into the operating room. I needed to know exactly what I had already done and what was the status of each patient. So I made a database in FileMaker Pro myself and kept track of everything, so that way I knew everything about every patient. Then I got a new job as head of health informatics research and I lucked out with a fantastic team of young programmers who took everything and developed it into a big database. The key element has always been the timeline.
The patient that was treated with the episcleral topotecan cup is under the name “Zoe Brave” in the demo database.
Explore DePICTRB for yourself: http://depictrb.technainstitute.com
Username: demo-user | Password: Demo1234
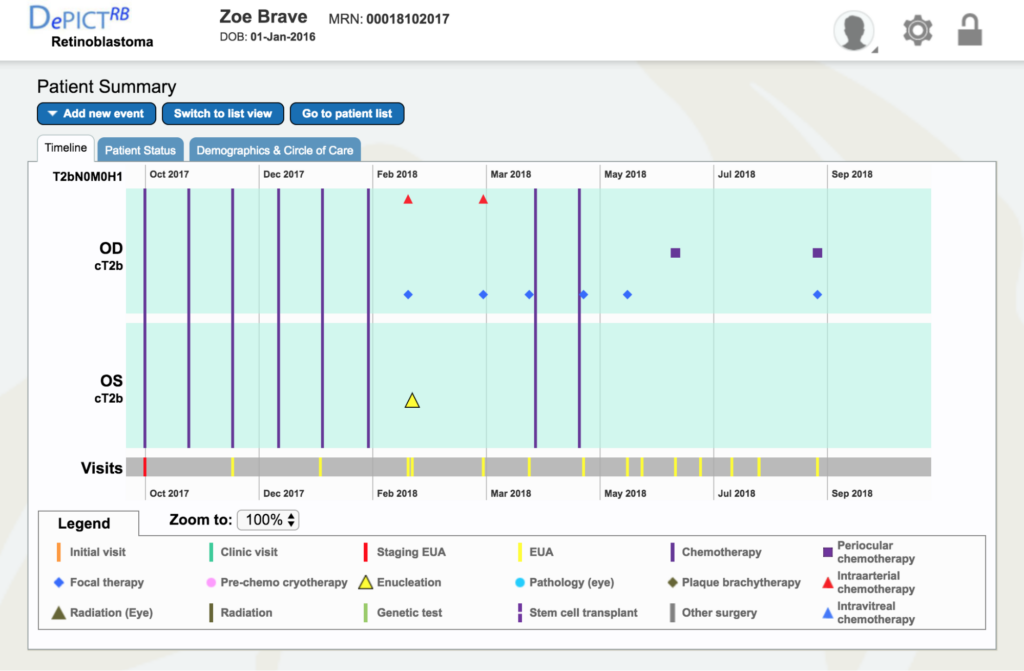
Dr. Gallie: Go to Zoe Brave, the 4th patient down. You can see immediately how much treatment she’s already had – six systemic chemotherapies for both eyes being the new staging clinical tumor, because they both had vitreous seeds and tumors all over. That’s basically the most severe kind of eye we would be trying to treat.
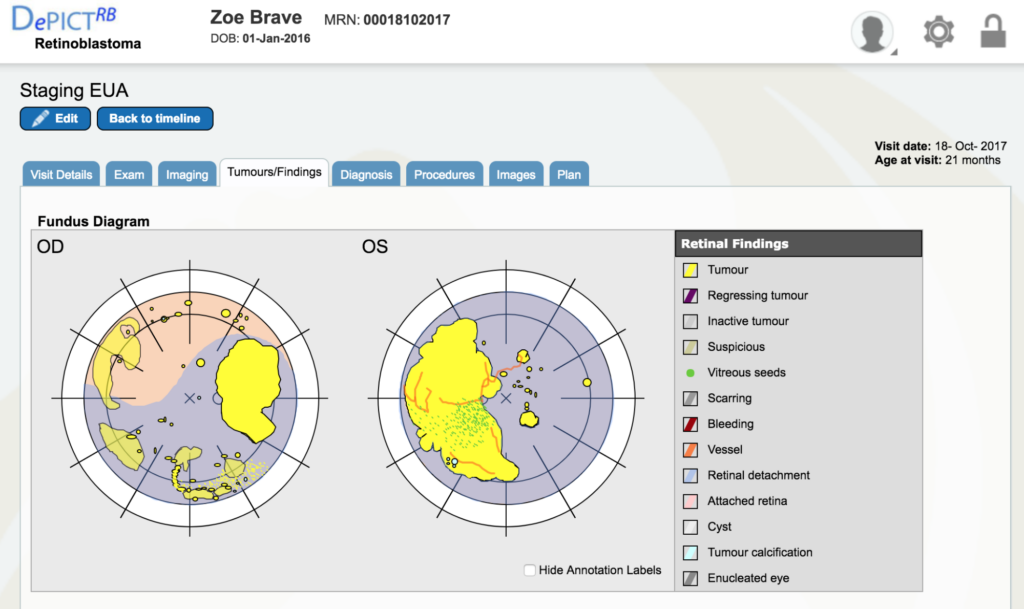
Dr. Gallie: Double click on the staging EUA (the bottom red line) for October 18th. It will take you right to that visit. Go to “tumors and finding” which are the retinal drawings. All the yellow is tumor and all the blue is retinal detachment, so you can see what a situation we had.
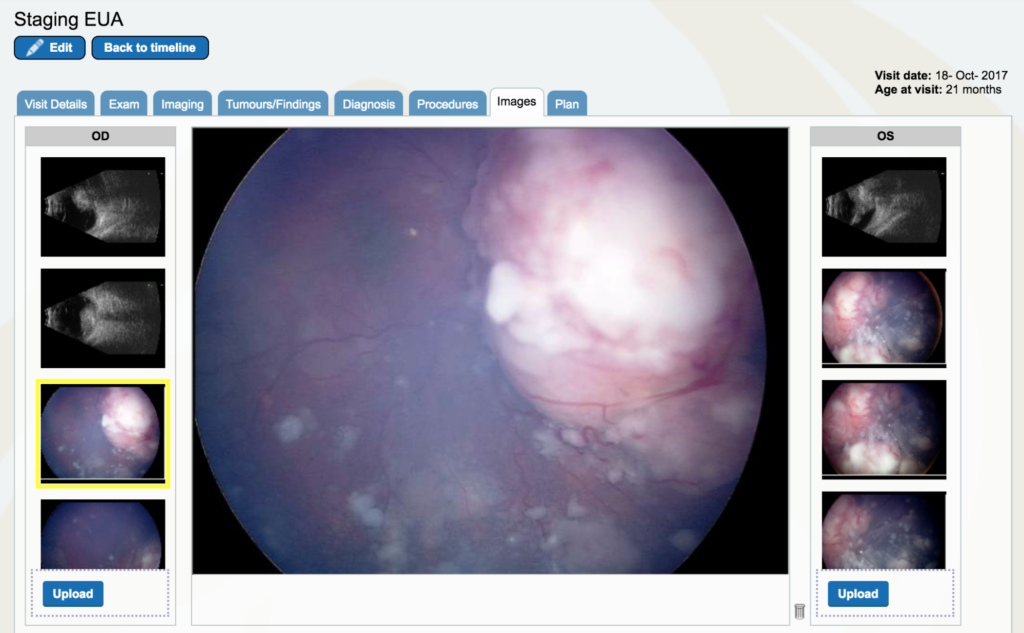
Dr. Gallie: The left eye (OS), if you look at the second image down, you can see it is even more severe. There is no optic nerve visible, the retina has folds, the tumor is everywhere – it’s a huge shamble. And that’s the eye we ultimately didn’t succeed with.
Dr. Gallie: So let’s go back up to timeline. Every single examination under anesthetic (EUA, all the yellow bars) have all that data behind them. The blue lines just have the data behind what the chemotherapy drug was used. They are not filled out well in this demo. In the real operational one, we will have all the details put in as soon as they happen.
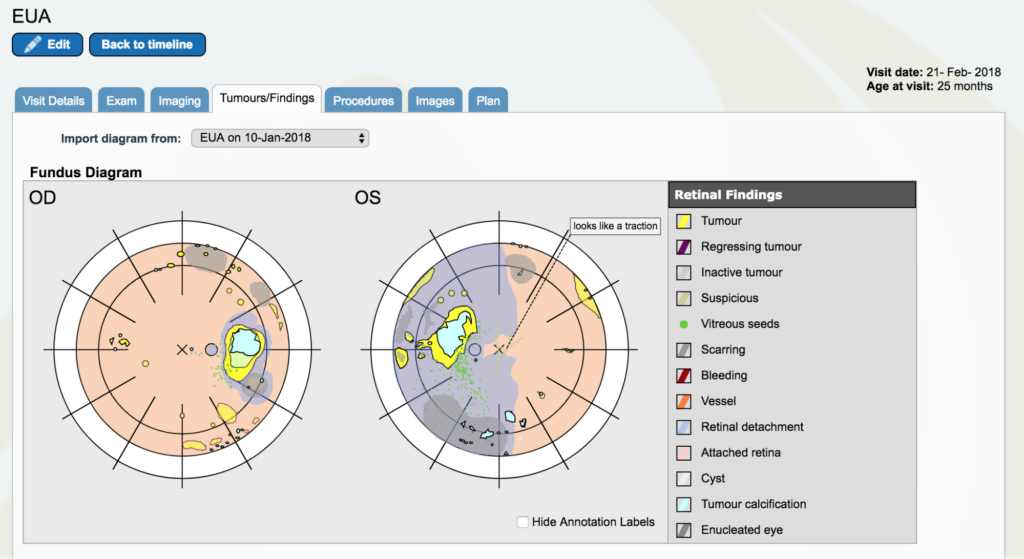
Dr. Gallie: Now, go to February 2018. There are two yellow lines very close together. Go to the first one, the 21st of February. And again, look at the “Tumors/Findings.” You see that we’ve done pretty well on the right eye in comparison. In fact, that eye had reasonably good vision. Whereas the other eye had really no vision and had still a large retinal detachment that actually had tumor all over the place. There is a particularly troublesome little tumor in the left upper corner of the eye as you’re looking at the left eye (OS) that comes to be a big problem later.
Dr. Gallie: At that point, go back to the timeline. That’s when we decided to take out the left eye because it actually had a little tumor on top of the nerve that we could see with optical coherence tomography. In fact, when we looked in that day (originally we planned to do more treatment), we looked in and we said, “No, there’s tumor on the nerve. Let’s take it out.” We took the patient under the same anesthetic back up into the OR and removed the eye, all in one day, changing the rest of our plan and schedule completely.
The red diamond above is the intra-arterial chemotherapy. So for the right eye, we then gave intra-arterial chemotherapy. We gave two doses for the right eye (labeled as the two red diamonds at the top) and the other eye is then removed.
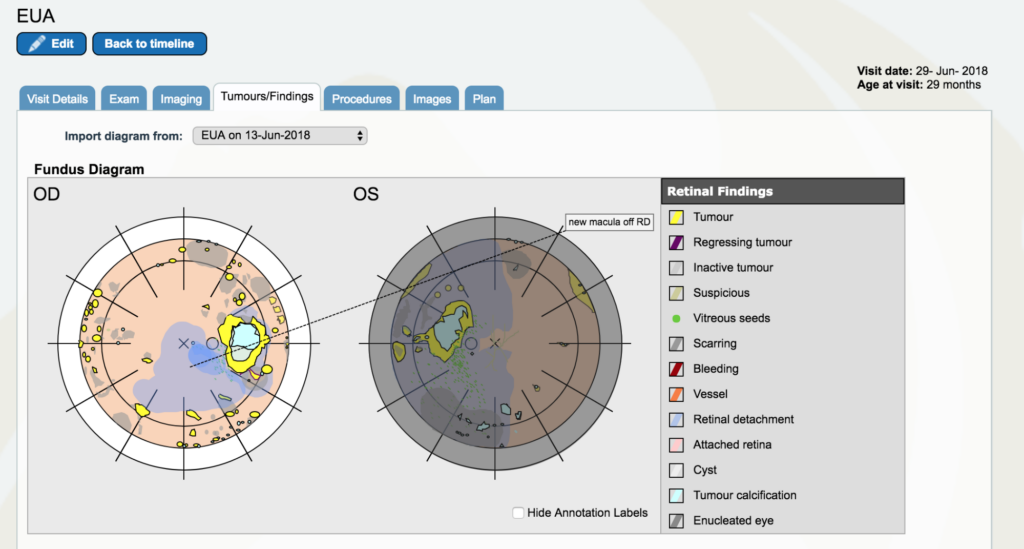
Dr. Gallie: So now go back between May and July. You’ll see a yellow line with June 29th on it. It’s right below the blue square for periocular therapy. Open that one up. Go to “tumors and findings”. Now, the left eye still shows the way the tumors look the day we took it out, but it’s covered in grey to show that it’s gone. It’s still shown there to remind people why we took it out, instead of just saying it’s gone. This is because it is an important part of the history of understanding and for the patient to know as well that that eye was dangerous at that moment we took it out.
In the other eye, now we have retinal detachment covering the fovea. The problem tumor that was always there that very first instance was hanging over the disc in the first pictures we looked at together. We have all these yellow dots and scars everywhere and we have been treating with laser. The next time we looked back, that one would be a scar and another one would be in its place. Remember how bad it looked in the first place – these were everywhere and now they are growing and none of our treatments are working.
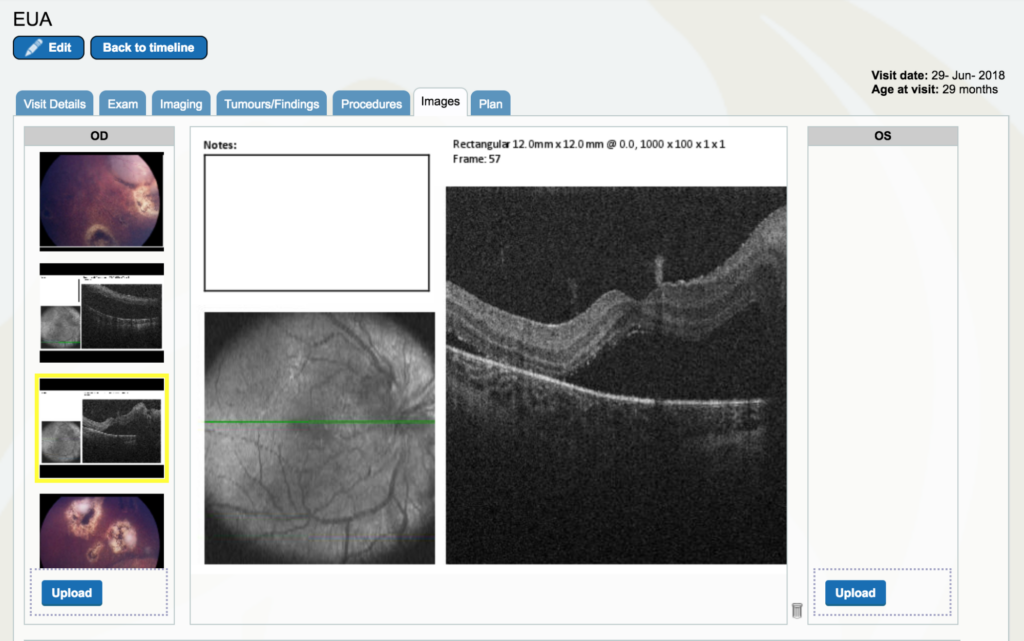

Dr. Gallie: Look at the “images”. If you go to the right eye, drag the cursor all the way down to near the bottom and you’ll start to see optical coherence tomography pictures.. Click on the second black and white photo. The green line is where the optical coherence tomography scan is done between the optic nerve and the center of vision. You don’t really see too much of the optic nerve, but it is rising up to the right. Then you see the fovea right in the middle and you see there is black space which is retinal detachment under the fovea. This eye had a little white fleck of calcium that is sitting right next to the fovea which is a really good landmark. Look at the green line right near the fovea, it’s barely seen, but you can see that so we know that is the fovea, right at the center of the fovea and that’s detached. That had not been detached before.
Dr. Gallie: So all of that led me to go off to a meeting in Barcelona where I met Linn Murphree and I was asking him what could we do with this child.
Let’s go back to the timeline and so we are now at June 29th. I went to a meeting in the middle of June, before June 29th, and talked to Linn Murphree and he told me what he had been working on for 20 years finally had FDA approval to use in a human subject. That was very exciting to me because I knew I had a patient that needed it, thinking of Zoe. We had been through a whirlwind of effort from the middle of June until the 29th when we actually had convinced Sick Kids Hospital and Health Canada. By 5 o’clock that evening, I had the episcleral device to put in the eye on the next day.
So I had it on the 28th at 5 o’clock with all the approvals, ready to roll. I spent a full week of work, rewriting, talking on the phone, showing images of the child’s eye and the terrible options which included taking out the last eye, his only eye, irradiating him which would give him a higher risk for second cancers, or putting a radioactive plaque under the tumor beside the optic nerve which would treat that but would not treat all the seeds that were everywhere and would damage his optic nerve so he would ultimately lose vision in the eye anyway. We did not have a very good space to do anything for him, so Linn Murphree told me he had a Phase I trial, which means testing for toxicity for something new, going on in LA but no patient had ever been treated with it. That made Health Canada more tentative, as Health Canada did not want us to be the first to put this in a patient’s eye. But I convinced them we should do it, I got their approval for the device and we put it in.
It’s a very simple, beautiful thing. It’s made of three things that have been in humans for 20 years, placed together in this neat little package that you attach on the outside of the eye, so it is really absurd to talk about toxicity in this in my opinion. We obviously need to do some studies putting it in and measuring everything you can think of in children to show it is not toxic, but it’s something to start really quickly doing because if it’s not toxic and if it’s effective, it could rescue this child’s eye and a lot of other children who are in a similar position and there are lots of them. In fact, I would have about five more that I would put it in tomorrow if they would let me.
So I would like to start a Phase I trial in Canada at SickKids. I want to do it only at SickKids so all the bureaucracy will not interfere, but that doesn’t mean I can’t treat patients from all over if they come to SickKids. We would do all of the imaging, and set up all the follow-ups and trial entry. The kids would go home with their little cup in, come back in 40 days, and we would take it out after we get things really well organized with people assisting us in other locations but not be actually initiating it in other centers so we don’t need their ethics approval. They could take it out wherever they are, so this is a trial I would like to start as soon as I can get it going.
But let me go back to this child from the database.
We put the episcleral cup in on day 0 and we took it out on day 70 which is longer than it was meant to be in and you’ll see the reason. The cup is a beautiful little thing about 10mm in diameter and has a handle that sticks out a little. It has topotecan in a poly carrier that all sorts of pills and things are made of in medicine so there is nothing new or dangerous in that. There’s nothing new in topotecan. We’ve injected it beside the eye in a gel before with some efficacy but it did not stay quite long enough. The cup stays as long as you want it. Then you put glue in a little groove there because you don’t want any glue between the sclera and the drug. Then we had the eyes held with these sutures so they are looking way down and out so we can get close to where the tumor is.
That was day 0.
By day 12, the tumor had already changed. All the little spots were gone except one and it was on the opposite side of the eye. The tumor was smaller and thinner but it was the only active tumor other than the big round one that was still remaining and by day 28 even it was gone and it has been all gone ever since.
On day 40 we were meant to take it out but we said it’s still changing, there is stillmore calcium showing, less tumor on top of it and we are actually seeing a little edge of scar there that we have never seen recently at all. And the black scar that would be totally covered up is now emerging and this blood has been there since day 12. So that’s called level 1 toxicity because there’s bleeding, but that bleeding to us was a really good sign because it means the tumor is dying. Dying tumors bleed when their blood vessels collapse.
Before we put the cup in, we had vitreous seeds. You see the snowballs here, they are greasy snowballs which means they are very actively growing and you can’t really see the optic nerve. On day 70, the optic nerve is clear and there are none of those there at all. The fovea was off, but by day 70 it is back. So finally on day 70 we took the cup out. We just picked up the handle and it fell out. We picked the carrier up and picked the glue up that was on the sclera, froze it all and now it is all ready to go back to California because they want to measure any topotecan that is still left. I have another child. I really want to have this one but I have to convince Health Canada, so I need to do a lot of work presenting all of this.
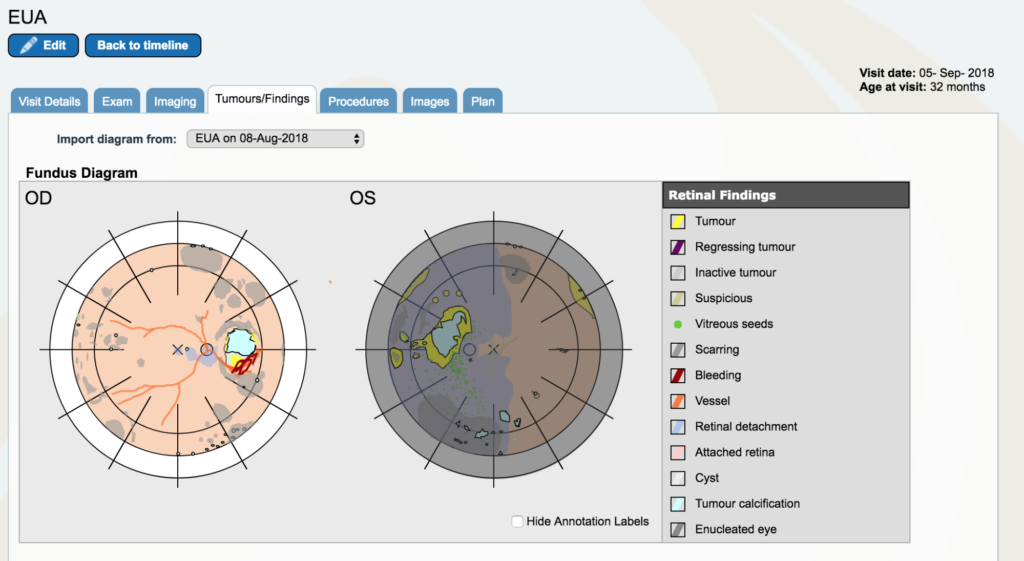
Dr. Gallie: Now let’s go back to September 5th. And look at “tumors and findings”. Pretty different story. So, the tumor itself is a lot smaller, it’s got a little edge that now we are starting to laser.
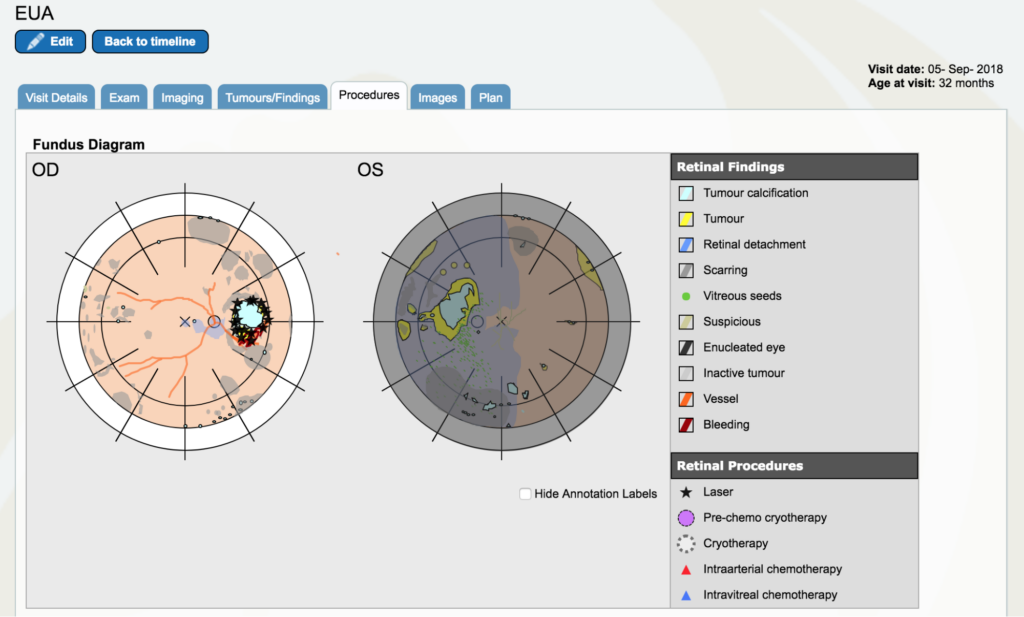
Dr. Gallie: I am going to go on “procedures” and I am going to put laser marks wherever I lasered. All of the residual tumor out here was lasered. But that’s only the beginning of lasering this because we do a little bit at a time, too much could cause complications. Because this is not towards the center vision, we can laser all the way around this. In the “procedures” tab, all the black stars are laser burns. We think the Topotecan cup was put right below that just in the corner of that, but we don’t indicate where it was put.
Q: Did you expect the results to be like this? What was your hope?
Personally, I thought there was a strong possibility that it would be this good. I didn’t say that loudly. I said loudly that there wasn’t going to be toxicity and I was totally convinced of that. My colleague who I love dearly, is a more conservative fellow, he’s a glass half empty person and I am a glass half full person. He’s now totally sold, of course, because of this very dramatic response!
So my hope is that in my proposed Phase I study we would, of course, get efficacy too, I would treat patients like this and patients with much less disease. I would also like to treat newborns.
